Discover our complete solutions portfolio covering Cathodic Protection and Corrosion management - PCB design and plating - Functional and decorative plating - Electrocoating - Acoustics
Discover Protect
Elsyca V-PIMS
A revolution in digital PIMS combining Pipeline Corrosion Integrity Management System (PIMS) and computational modeling capabilities
Elsyca IRIS
Deep analysis of AC threats supporting efficient mitigation systems computer-aided design
Elsyca CatPro
Graphical simulation platform for cathodic protection and DC stray current analysis of pipeline networks
Elsyca CPManager
3D CAD-based software simulation platform for the computer-aided design and analysis of cathodic protection installations
Elsyca ACTA
Unique solution offering accurate, disambiguated, and tailored risk ranking report of pipeline networks
Plate
Elsyca PlatingManager
Leverage a digital twin of your plating line to predict plating performance and increase manufacturing capacity
Elsyca PCBBalance
The world’s only PCB DFM software that applies automated and optimized copper balancing to your PCB design and panel layout.
Elsyca PCBPlate
State-of-the-art graphical simulation platform for enhancing the plating performance of your PCB panel and pattern plating processes.
Elsyca ECoatMaster
CAD independent software platform for the simulation of the automotive electrocoating process of a body-in-white (BIW).
Elsyca EPOS
Simulate the performances of electropolishing processes based on a virtual mock-up of the electropolishing cell.
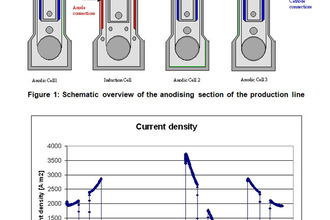
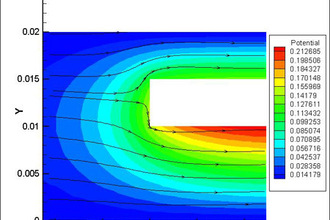
Elsyca AnodizingManager
State-of-the-art graphical simulation platform for analyzing the production performance and quality of anodizing processes.
Innovate
Elsyca CorrosionMaster
CorrosionMaster identifies corrosion hot spots and predicts corrosion rates, enabling engineers to look at alternative material combinations and/or coating systems, or investigate corrosion-mitigating measures.
Elsyca LeakageMaster
Improve vehicles interior acoustic comfort by performing upfront virtual smoke tests.
Elsyca MeshingMaster
Automatically creates meshes for a variety of applications such as acoustics, CFD, thermal analysis, etc
Elsyca XPlorer
Interactive simulation results viewer for Finite Elements results
Elsyca XPlorer3D
Analyze, Understand and Get Immersed in your results
Anodizing
Surface Finishing Solutions Overview
Download file
Ensure even growth of the metal oxide layer
Increase production speed while maintaining the final quality of your products
Overcome the challenges related to non-uniform heat generation and current distribution to increase the production efficiency of your continuous reel-to-reel anodizing processes. Elsyca's solution for anodizing operations support manufacturers of various industries, from aerospace to consumer electronics to:
- analyze and optimize the most optimal rack design and layout for better productivity;
- control the pore growth and diameter for improved sealing processes;
- validate the anodizing tank infrastructure in combination with the processed product geometry for the most efficient productivity;
- ensure a top visual appearance and functional properties;
- increase line speed by up to 10% and decrease total power consumption by up to 20%.
Control upfront the metal oxide layer growth and optimize your process parameters to speed-up your line operations by up to 10% while reducing your power consumption.
Interested in knowing more about our solutions for the anodizing process optimization?
Download our surface finishing solutions overview